A research team from the University of Wisconsin-Madison, led by physicist Matthew Pernin, has determined that the Warburg Effect could be a key factor in cancer cells. The team’s research, which has been published in the Journal of the Society for Experimental Biology, could lead to a new way to treat cancer with a drug.
In 1954, Oncology professor Otto Warburg presented his theory which showed that cancer cells thrive in anaerobic environments. How can anaerobes exist in an anaerobic environment? They don’t, but they resemble anaerobic environments. This was called the “Warburg Effect” because he said that cancer cells are powered by fermentation.
Professor Warburg also proposed that normal cells could become cancerous if they switched from anaerobic to aerobic metabolism (like many cancer cells do). The Warburg Effect, a metabolic reaction responsible for generating cancerous tumors, was discovered in the mid-20th century.
We know that within the mitochondria, the cell’s energy-producing organelles, abnormal cell growth is a crucial step in the development of cancer. However, the underlying reasons for this are far from well understood. One of them is the Warburg effect.
The Warburg effect refers to the fact that cancer cells prefer fermentation over the more efficient mitochondrial route of oxidative phosphorylation as an energy source (OxPhos). This was discussed in a prior article.
Cells in normal tissues may utilize OxPhos, which produces 36 ATP, or anaerobic glycolysis, which produces 2 ATP. Anaerobic refers to the absence of oxygen, whereas glycolysis refers to the burning of glucose. Using oxygen in the mitochondria produces 18 times more energy than anaerobic glycolysis for the same molecule of glucose. When normal tissues run out of oxygen, such as muscles during a race, they choose the less efficient pathway. Lactic acid is produced, causing a burning feeling in the muscle.
Cancer, on the other hand, is a whole other story. It utilizes a less efficient form of energy generation even when there is oxygen present (i.e. aerobic as opposed to anaerobic) (glycolysis rather than phosphorylation). Why is it present in nearly all tumors? Given the abundance of oxygen, this seems wasteful, since OxPhos can generate much more ATP.
But it can’t be that dumb, since it’s seen in virtually every cancer cell throughout history. As previously said, this finding is so remarkable that it has become one of the new hallmarks of cancer. But why is that? When something seems irrational yet nevertheless occurs, it generally indicates that we don’t comprehend it. As a result, rather than dismissing it as a natural occurrence, we should attempt to comprehend it.
There is an evolutionary push on single-celled organisms, such as bacteria, to reproduce and expand as long as resources are available. Consider the yeast cell on a piece of bread. It’s exploding in size. On a dry surface, such as a table top, yeast stays dormant. There are two major factors that influence development.

Not only does development require energy, but it also necessitates the initial building bricks. Take a look at a home on the outskirts of town. You’ll need more than simply bricks; you’ll also need constructors. Cells, too, need fundamental materials (nutrients) to develop.
Many nutrients are typically in circulation in multicellular organisms. A liver cell, for example, finds nutrients all over the place. Because the liver only takes up these nutrients when it is stimulated by growth hormones, it does not expand. There are many bricks in our building example, but the foreman ordered the builders not to build. That is why no new structures are being constructed.
According to one hypothesis, the Warburg effect is used by cancer cells not just to generate energy but also to acquire the substrate required for their development. A cancer cell requires numerous cell components to divide, which in turn need building blocks such as acetyl-CoA, which may be used to make other tissues such as amino acids and lipids.
Palmitate, a key component of the cell wall, for example, needs 7 ATP and 16 carbs, which may be produced from 8 acetyl-CoA. Because OxPhos is fully burnt for energy, it produces a lot of ATP but very little acetyl-CoA.
As a result, if all of the glucose was burnt for energy, there would be no more building blocks available to create new cells. One glucose molecule delivers 5 times the energy in palmitate, but it takes 7 glucose molecules to create the building blocks. Pure energy generation is not beneficial for a growing cancer cell’s growth. Instead, aerobic glycolysis will optimize growth and multiplication by generating both energy and substrate.
This is useful in a closed environment, but cancer does not develop in a petri dish. Nutrients, on the other hand, are seldom a limiting factor in the human body; glucose and amino acids are plentiful. There is no selection pressure to increase ATP production since energy and building materials are abundant.
It’s conceivable that cancer cells utilize part of the glucose for energy while others are used for biomass expansion. It may make sense to utilize part of the money for the bricks and some for the builders in an isolated system. The human body, on the other hand, is not such a system. A breast cancer cell, for example, has access to the circulation, which provides glucose for energy as well as amino acids and lipids for cell growth.
It’s also pointless to nail down obesity when there are so many factors involved. Because it can readily acquire the building components, the cancer should utilize as much glucose as possible as an energy source in this scenario. This raises the issue of whether this interpretation of the Warburg effect contributes to cancer formation.
However, there is an intriguing implication. What should you do if your nutritional reserves are depleted? To put it another way, if we can activate our food sensors to indicate low energy levels, the cell is preferentially pushed to increase energy synthesis (ATP) and shift away from the cancer-promoting aerobic glycolysis. If insulin and mTOR levels are lower but AMPK levels are higher. Fasting is a simple dietary modification that may help with this. A ketogenic diet reduces insulin levels while activating mTOR and AMPK, two additional food sensors.
Glutamine
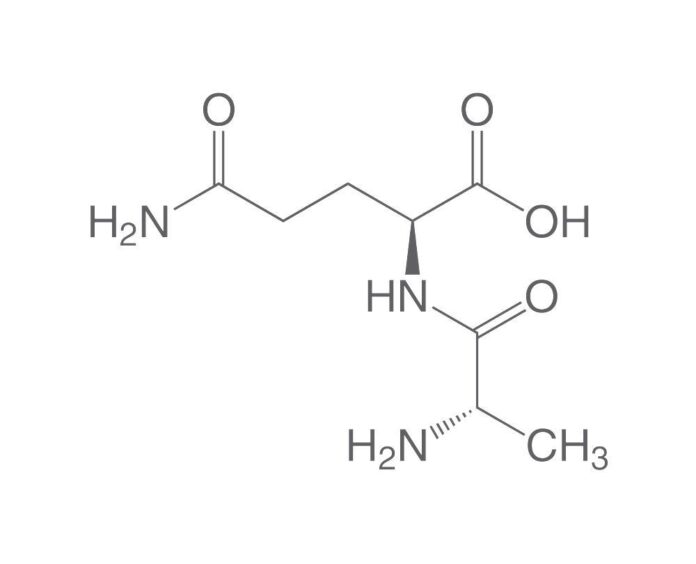
The Warburg effect is sometimes misunderstood in the sense that cancer cells can only utilize glucose. That is not the case. A mammalian cell can catabolize two main molecules: glucose and the protein glutamine. Glucose and glutamine metabolism are both disrupted in cancer patients.
Many tumors seem to rely on glutamine to live and grow since it is the most prevalent amino acid in the blood. Particle emission tomography (PET) is the best way to see this impact (PET). PET scans are a common kind of imaging in oncology. The body is injected with a tracer. Fluorine-18-fluorodeoxyglucose (FDG) is a kind of normal glucose that has been tagged with a radioactive material that the PET scanner can detect.
Most cells only take in a little amount of glucose on a daily basis. Cancer cells, on the other hand, sip glucose like a camel after a desert journey. These glucose-labeled cells may be seen in malignant tissues and are thought to represent active cancer development sites.
In this case of lung cancer, a significant region of the lung absorbs glucose at a rapid rate. This indicates that cancer cells have a significantly higher glucose need than normal tissues. However, utilizing glutamine, a radioactively tagged amino acid, there is another method to conduct a PET scan. This indicates that glutamine is treated with the same respect by certain crustaceans. Some tumors, in fact, cannot live without glutamine and seem to be glutamine-dependent.
In the 1930s, Warburg discovered cancer cells with abnormal glucose metabolism, but it wasn’t until 1955 that Harry Eagle observed that certain cells in culture consumed 10 times more glutamine than other amino acids. This is also true for many cancer cell lines, according to more recent studies from the 1970s. Other studies have shown that glutamine is converted to lactate, which seems to be a waste.
The glutamine was transformed to lactate, a waste product, rather than being used for energy. This is the same inefficient process that occurs when glucose is consumed. The tumour switched from glucose to lactate and didn’t get the full advantage of each molecule’s energy source. Glucose supplies acetyl-CoA to the mitochondria, while glutamine delivers oxaloacetate to glutamine (see graph). In the first stage of the TCA cycle, this supplies the carbon for citrate synthesis.
Glutamine shortage seems to be especially harmful to certain tumors. In the absence of glutamine, pancreatic cancer, glioblastoma multiforme, and acute myeloid leukemia frequently die in vitro. The common misconception is that a ketogenic diet would deprive cancer of glucose. In certain tumors, glutamine is a more significant component.
What makes glutamine so unique? The primary regulator of protein synthesis, the mTOR 1 complex, mTORC1, reacts to glutamine levels, which is a significant finding. Growth factor signaling is mediated via the insulin-like growth factor (IGF)-PI3K-Akt pathway in the presence of adequate amino acids.
This PI3K signaling pathway is important for controlling growth and glucose metabolism, demonstrating the tight link between growth and nutrition and energy availability once again. If there are no nutrients, the cells will not grow.
This is evident in the research of oncogenes, the majority of which are regulated by tyrosine kinases. The control of glucose metabolism is a typical characteristic of tyrosine kinase signaling linked to cell growth. This does not happen in regular, non-multiplying cells. The MYC oncogene is very susceptible to glutamine deficiency.
Here’s what we know thus far. Cells of cancer:
- Even when oxygen is abundantly accessible, switching from a more efficient method for OxPhos energy generation to a less efficient one.
- Glucose is required, as well as glutamine.
The million-dollar issue, though, remains. Why? This occurrence is much too widespread to be a coincidence. Cancer is caused by a variety of causes, including viruses, ionizing radiation, and carcinogenic substances (tobacco, asbestos), among others. There is no pure dietary treatment if it is not a basic dietary issue. The following theory seems to me to be the most reasonable. Because it cannot, the cancer cell does not choose a more efficient path.
When mitochondria are damaged or age, cells automatically seek other energy sources. To live, the cells are forced to convert to the phylogenetically old pathway of aerobic glycolysis. Now we’ll look at several atavistic cancer ideas.
– Jason Fung, M.D.

The Warburg Effect, a term coined by the Nobel Laureate Otto Warburg in the 1940s, refers to the uncontrolled cellular proliferation in cancerous tumors. This is due to the inefficient use of the energy in the metabolic processes of the cell, leading to an overproduction of anabolic intermediates (lipids, proteins, nucleic acids, etc.).
Meanwhile, the blood-borne oxygen available to the tumor cells is not sufficient to support all of these metabolic processes, leading to a lack of oxygenation of the cell and its inefficient delivery of oxygen to the tumor cells. This is what is known as the Warburg Effect as well.. Read more about why does the warburg effect happen and let us know what you think.
Frequently Asked Questions
Why does Warburg affect cancer?
Warburg effect is a phenomenon in which the production of ATP, the main energy currency of cells, is inhibited. The Warburg effect is seen in many different types of cancerous cells.
What is the Warburg effect and why is it a cancer hallmark?
The Warburg effect is a metabolic process in which cells use anaerobic glycolysis to generate ATP, instead of oxidative phosphorylation. This is generally seen as a hallmark of cancer cells.
What are the main mechanisms of the Warburg effect?
The Warburg effect is the phenomenon of glycolysis occurring in cancer cells. It is a metabolic process that occurs when glucose, which is present in the bloodstream, enters the cell and produces energy for the cell to use.